Abstract
Background: Reduced or low-dose direct oral anticoagulants (DOACs) have been studied in randomized control trials for the extended prevention of venous thromboembolism (VTE) after 6 months of treatment at the therapeutic doses. The real-world effectiveness as well as utilization of this approach in clinical practice compared to continuing at therapeutic doses is unknown.
Aims: Evaluate the efficacy of reduced-dose DOACs compared to standard therapeutic anticoagulation in patients with extended anticoagulation.
Methods: Consecutive patients with VTE were identified using the Mayo Clinic VTE Registry from March 1st , 2013 to December 31st, 2020. Patients were followed prospectively for outcomes of VTE recurrence, death, major bleeding and clinically relevant non major bleeding (CRNMB) either in person or by survey. Type of anticoagulation and dose changes were recorded, and progressing to a low dose was treated as an endpoint. In the models relating it to the outcome, it was treated as a time-dependent covariate in the cox models. After completing standard anticoagulation treatment for 3 months with any anticoagulant, patients continuing anticoagulation were divided into two groups: standard therapeutic anticoagulation (any drug) vs those who transitioned to rivaroxaban 10 mg daily or apixaban 2.5mg twice daily any time after 3 months. Patients with recurrent VTE, major or CRNMB during the first 3 months were excluded from further analysis.
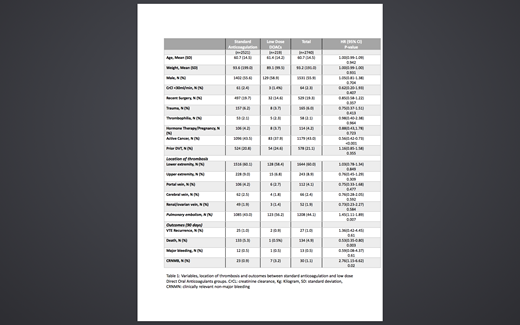
Results: 219 patients (153 on Apixaban and 66 on Rivaroxaban) were identified in the low dose DOAC and 2521 in the standard anticoagulation group. The mean age was 60.7 years, mean weight was 93.2 Kg and 55.9% were males (Table 1). Patients on low dose DOACs were about the same age and weight compared to standard anticoagulation group. Among all diagnosis categories, patients with active cancer were less likely to be prescribed low dose DOACs (HR 0.56; 95%CI 0.42-0.73; P<0.001), while low dose DOACs prescription was more likely in patients with pulmonary embolism (HR 1.45; 95%CI 1.11-1.89; P=0.007). The median months to start a low dose DOAC after completing the 3 months was 3.1 months with standard deviation of 6.6 months and interquartile range 2.8 months. There was no statistically significant association in the likelihood of going to a low dose group in relation to VTE recurrence (HR 1.36; 95%CI 0.42-4.45; P=0.61) or major bleeding (HR 0.59; 95%CI 0.08-4.37; P=0.61). However, transitioning to low dose DOAC was associated with reduced mortality (HR 0.53; 95%CI 0.35-0.80; P=0.003). Also, patients on low dose DOACs were more likely to have a CRNMB (HR 2.76; 95%CI 1.15-6.62; P=0.02).
Conclusion: In this study of patients continuing anticoagulation past 3 months for an initial acute venous thromboembolic event, transitioning to low dose DOACs was not associated with any statistically significant association with VTE recurrence or major bleeding. However, mortality was lower and CRNMB was higher in this unadjusted analysis. Our results show that physicians are not always waiting until 6 months to transition to low doses and low doses are being utilized in cancer patients. Further research is needed to better understand the higher risk for CRNMB identified in this group.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal